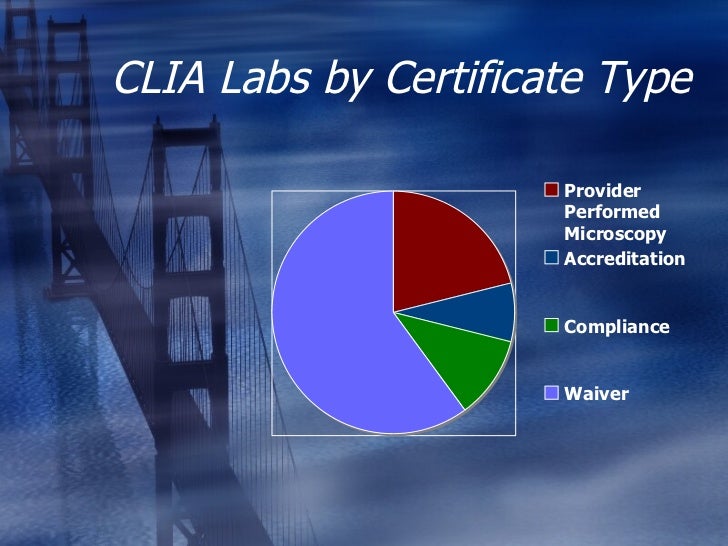
Clia Brochures
Clia Brochures - Review policies, procedures and processes; Documented competency assessment is required for individuals fulfilling the following personnel responsibilities outlined in subpart m of the clia regulations: Brochures to help explain the clinical laboratory improvement amendments (clia) regulation requirements are listed below in the downloads section. The clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards applicable to all u.s. Clia established quality standards for laboratories to ensure the accuracy, reliability, and timeliness of patient test results regardless of where the test is Through the clinical laboratory improvement amendments (clia) program, cms regulates all lab testing (with some specific exceptions and state exemptions) done on humans in the u.s. Brochures to help explain the clinical laboratory improvement amendments (clia) regulation requirements are listed below in the downloads section. The clinical laboratory improvement amendments (clia) program regulates laboratories that test human specimens and ensures laboratories produce accurate, reliable, and timely patient. This brochure provides information on the clinical laboratory improvement amendment’s (clia) new quality control option called individualized quality control plan, also known as iqcp. Facilities or sites that test human specimens for. Through the clinical laboratory improvement amendments (clia) program, cms regulates all lab testing (with some specific exceptions and state exemptions) done on humans in the u.s. The clinical laboratory improvement amendments of 1988 (clia) statute revised the federal program for certification and oversight of clinical laboratory testing to ensure accurate, reliable. Laboratory director responsibilities (pdf) clia. Brochures to help explain the clinical laboratory improvement amendments (clia) regulation requirements are listed below in the downloads section. Program to regulate laboratories that perform testing on patient specimens to ensure accurate and reliable test. The clinical laboratory improvement amendments (clia) program regulates laboratories that test human specimens and ensures laboratories produce accurate, reliable, and timely patient. Brochures to help explain the clinical laboratory improvement amendments (clia) regulation requirements are listed below in the downloads section. Facilities or sites that test human specimens for. Documented competency assessment is required for individuals fulfilling the following personnel responsibilities outlined in subpart m of the clia regulations: Review policies, procedures and processes; The clinical laboratory improvement amendments of 1988 (clia) statute revised the federal program for certification and oversight of clinical laboratory testing to ensure accurate, reliable. The clinical laboratory improvement amendments (clia) program regulates laboratories that test human specimens and ensures laboratories produce accurate, reliable, and timely patient. Understand the laboratory director delegations and monitor them. Clia established quality standards for. The clinical laboratory improvement amendments (clia) program regulates laboratories that test human specimens and ensures laboratories produce accurate, reliable, and timely patient. Brochures to help explain the clinical laboratory improvement amendments (clia) regulation requirements are listed below in the downloads section. Understand the laboratory director delegations and monitor them. Clia established quality standards for laboratories to ensure the accuracy, reliability,. Review policies, procedures and processes; Laboratory director responsibilities (pdf) clia. Documented competency assessment is required for individuals fulfilling the following personnel responsibilities outlined in subpart m of the clia regulations: Program to regulate laboratories that perform testing on patient specimens to ensure accurate and reliable test. This brochure explains the requirements and procedures for verifying the accuracy, precision, and other. Through the clinical laboratory improvement amendments (clia) program, cms regulates all lab testing (with some specific exceptions and state exemptions) done on humans in the u.s. Laboratory director responsibilities (pdf) clia. Clia provides regulatory standards and certificates for clinical laboratory testing in facilities that test human specimens for diagnostic, preventive, or therapeutic purposes, and for health. Brochures to help explain. Program to regulate laboratories that perform testing on patient specimens to ensure accurate and reliable test. Clia provides regulatory standards and certificates for clinical laboratory testing in facilities that test human specimens for diagnostic, preventive, or therapeutic purposes, and for health. Understand the laboratory director delegations and monitor them. The clinical laboratory improvement amendments (clia) establishes a. Facilities or sites. Brochures to help explain the clinical laboratory improvement amendments (clia) regulation requirements are listed below in the downloads section. The clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards applicable to all u.s. This brochure provides information on the clinical laboratory improvement amendment’s (clia) new quality control option called individualized quality control plan, also known as iqcp. The. Understand the laboratory director delegations and monitor them. Review policies, procedures and processes; This brochure provides information on the clinical laboratory improvement amendment’s (clia) new quality control option called individualized quality control plan, also known as iqcp. The clinical laboratory improvement amendments (clia) program regulates laboratories that test human specimens and ensures laboratories produce accurate, reliable, and timely patient. The. The clinical laboratory improvement amendments (clia) program regulates laboratories that test human specimens and ensures laboratories produce accurate, reliable, and timely patient. The clinical laboratory improvement amendments (clia) program regulates laboratories that test human specimens and ensures laboratories produce accurate, reliable, and timely patient. This brochure explains the requirements and procedures for verifying the accuracy, precision, and other characteristics of. Program to regulate laboratories that perform testing on patient specimens to ensure accurate and reliable test. Clia established quality standards for laboratories to ensure the accuracy, reliability, and timeliness of patient test results regardless of where the test is Understand the laboratory director delegations and monitor them. The clinical laboratory improvement amendments of 1988 (clia) statute revised the federal program. Documented competency assessment is required for individuals fulfilling the following personnel responsibilities outlined in subpart m of the clia regulations: Brochures to help explain the clinical laboratory improvement amendments (clia) regulation requirements are listed below in the downloads section. Through the clinical laboratory improvement amendments (clia) program, cms regulates all lab testing (with some specific exceptions and state exemptions) done. Clia established quality standards for laboratories to ensure the accuracy, reliability, and timeliness of patient test results regardless of where the test is Brochures to help explain the clinical laboratory improvement amendments (clia) regulation requirements are listed below in the downloads section. This brochure explains the requirements and procedures for verifying the accuracy, precision, and other characteristics of test systems used in clinical laboratories under clia. The clinical laboratory improvement amendments (clia) establishes a. Facilities or sites that test human specimens for. The clinical laboratory improvement amendments (clia) program regulates laboratories that test human specimens and ensures laboratories produce accurate, reliable, and timely patient. Review policies, procedures and processes; Through the clinical laboratory improvement amendments (clia) program, cms regulates all lab testing (with some specific exceptions and state exemptions) done on humans in the u.s. The clinical laboratory improvement amendments of 1988 (clia) statute revised the federal program for certification and oversight of clinical laboratory testing to ensure accurate, reliable. The clinical laboratory improvement amendments of 1988 (clia) regulations include federal standards applicable to all u.s. Program to regulate laboratories that perform testing on patient specimens to ensure accurate and reliable test. Laboratory director responsibilities (pdf) clia. The clinical laboratory improvement amendments (clia) program regulates laboratories that test human specimens and ensures laboratories produce accurate, reliable, and timely patient. This brochure provides information on the clinical laboratory improvement amendment’s (clia) new quality control option called individualized quality control plan, also known as iqcp. Documented competency assessment is required for individuals fulfilling the following personnel responsibilities outlined in subpart m of the clia regulations:CLIA Regulations Pertaining To Your Lab Quality Assurance Food And
CLIA Brochure 2 Regulations PDF Accuracy And Precision Reference
Guides on Updated CLIA Proficiency Testing Regulation
(CLIA) ID Requirement Policy
How Obtain CLIA Certificate Brochure PDF Health Sciences Medicine
The CLIA Compliance Reference Guide for Laboratory Managers
Understanding CLIA Your Guide to Lab Regulations Healthcare Online
CLIA Compliance
Through The Clinical Laboratory Improvement Amendments (Clia) Program, Cms Regulates All Lab Testing (With Some Specific Exceptions And State Exemptions) Done On Humans In The U.s.
Clia Provides Regulatory Standards And Certificates For Clinical Laboratory Testing In Facilities That Test Human Specimens For Diagnostic, Preventive, Or Therapeutic Purposes, And For Health.
Brochures To Help Explain The Clinical Laboratory Improvement Amendments (Clia) Regulation Requirements Are Listed Below In The Downloads Section.
Understand The Laboratory Director Delegations And Monitor Them.
Related Post: