Inari Clottriever Brochure
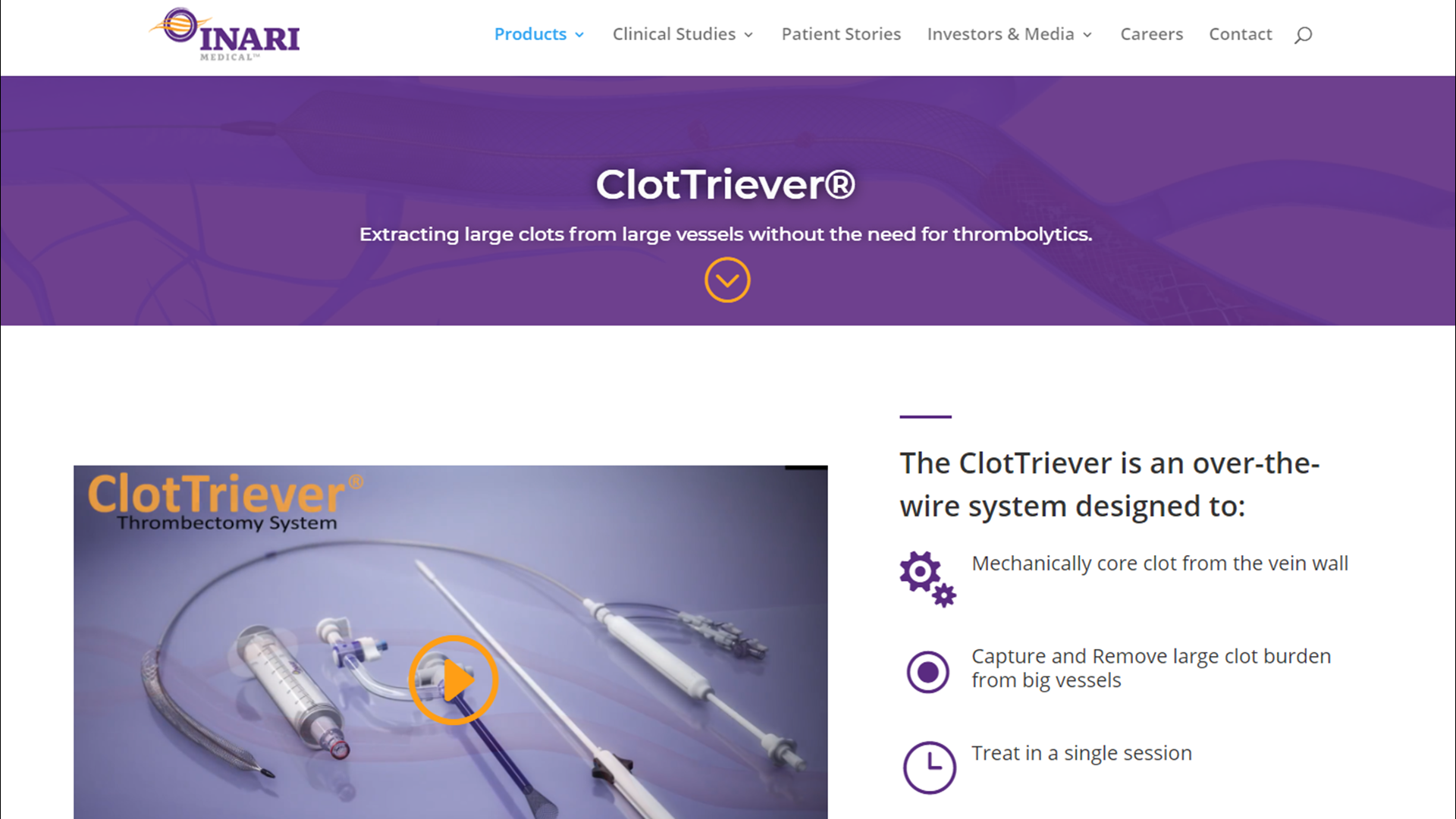
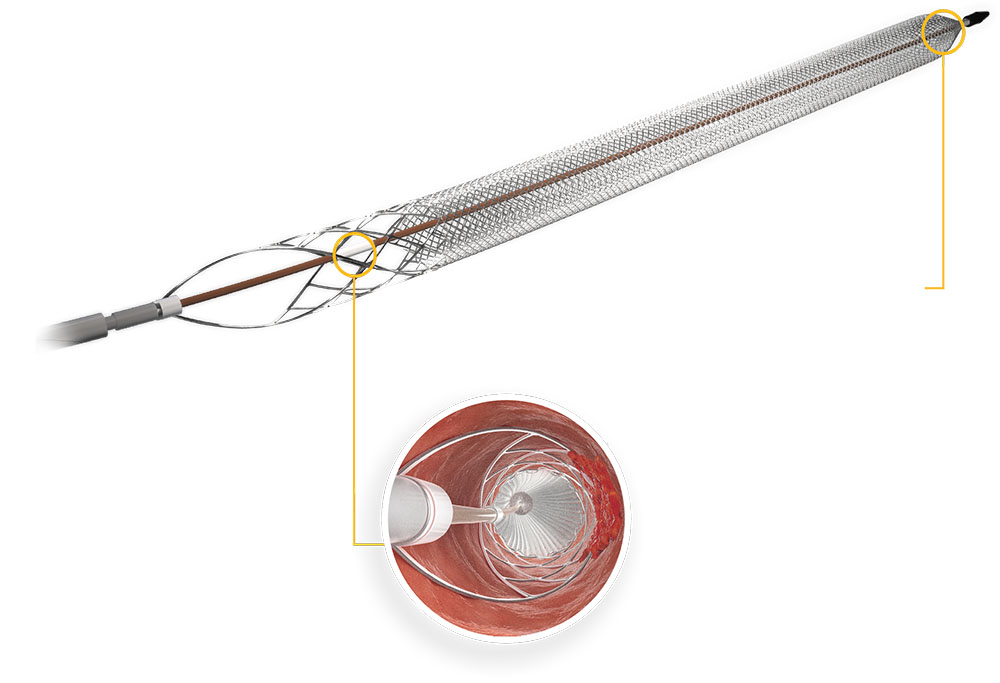
Inari Clottriever Brochure - In addition, it is designed to give physicians advanced control in chronic. • mechanically core clot from vein wall • capture and remove large clot burden from big vessels • treat in a single session • eliminate. 2) 2) injection, infusion, and/or aspiration of. The clottreiver® system by inari medical® is available to treat the full range of acute and chronic deep vein thrombosis. Inari medical announces first patient treated with the clottriever™ thrombectomy system; The clottriever® system is a medical device fda cleared for the treatment of deep vein thrombosis. The clottriever® system • designed to core, capture and remove large clots from large venous vessels • in february of 2017, the clottriever system received 510(k). The clottriever® catheter is a purely mechanical treatment option that liberates thrombus from the vessel walls and captures in a collection bag to be extracted via the clottriever® sheath. The clottriever thrombectomy system is intended for use in the peripheral vasculature including deep vein thrombosis (dvt). The inari clottriever is a percutaneous mechanical thrombectomy system consisting of an expandable nitinol collection bag that is dragged along the vein wall,. In addition, it is designed to give physicians advanced control in chronic. The inari clottriever is a percutaneous mechanical thrombectomy system consisting of an expandable nitinol collection bag that is dragged along the vein wall,. The clottriever thrombectomy system is intended for use in the peripheral vasculature including deep vein thrombosis (dvt). The clottriever® catheter is a purely mechanical treatment option that liberates thrombus from the vessel walls and captures in a collection bag to be extracted via the clottriever® sheath. As the first mechanical thrombectomy system to receive fda 510 (k) clearance for the treatment of pulmonary embolism, the flowtriever system is designed for rapid thrombus removal and. The clottriever® system is a medical device fda cleared for the treatment of deep vein thrombosis. The clottriever thrombectomy system is indicated for: The clottriever® system • designed to core, capture and remove large clots from large venous vessels • in february of 2017, the clottriever system received 510(k). Inari medical announces first patient treated with the clottriever™ thrombectomy system; Fda 510(k) clearance received for peripheral thrombectomy by inari | apr 10, 2017 | news The inari clottriever is a percutaneous mechanical thrombectomy system consisting of an expandable nitinol collection bag that is dragged along the vein wall,. See instructions for use for complete indications for use,. 2) 2) injection, infusion, and/or aspiration of. As the first mechanical thrombectomy system to receive fda 510 (k) clearance for the treatment of pulmonary embolism, the flowtriever system. The inari clottriever is a percutaneous mechanical thrombectomy system consisting of an expandable nitinol collection bag that is dragged along the vein wall,. The clottriever® catheter is a purely mechanical treatment option that liberates thrombus from the vessel walls and captures in a collection bag to be extracted via the clottriever® sheath. The clottriever thrombectomy system is indicated for: •. The clottriever® system is a medical device fda cleared for the treatment of deep vein thrombosis. See instructions for use for complete indications for use,. The clottreiver® system by inari medical® is available to treat the full range of acute and chronic deep vein thrombosis. The clottriever thrombectomy system is intended for use in the peripheral vasculature including deep vein. In addition, it is designed to give physicians advanced control in chronic. The clottriever® system • designed to core, capture and remove large clots from large venous vessels • in february of 2017, the clottriever system received 510(k). The clottriever® system is a medical device fda cleared for the treatment of deep vein thrombosis. Inari medical announces first patient treated. 2) 2) injection, infusion, and/or aspiration of. • mechanically core clot from vein wall • capture and remove large clot burden from big vessels • treat in a single session • eliminate. The clottriever thrombectomy system is indicated for: The clottriever® system is a medical device fda cleared for the treatment of deep vein thrombosis. The clottriever® system • designed. 2) 2) injection, infusion, and/or aspiration of. The clottriever thrombectomy system is intended for use in the peripheral vasculature including deep vein thrombosis (dvt). The clottriever® system • designed to core, capture and remove large clots from large venous vessels • in february of 2017, the clottriever system received 510(k). In addition, it is designed to give physicians advanced control. Inari medical announces first patient treated with the clottriever™ thrombectomy system; See instructions for use for complete indications for use,. In addition, it is designed to give physicians advanced control in chronic. 2) 2) injection, infusion, and/or aspiration of. • mechanically core clot from vein wall • capture and remove large clot burden from big vessels • treat in a. The clottriever® system is a medical device fda cleared for the treatment of deep vein thrombosis. In addition, it is designed to give physicians advanced control in chronic. • mechanically core clot from vein wall • capture and remove large clot burden from big vessels • treat in a single session • eliminate. As the first mechanical thrombectomy system to. The clottriever® catheter is a purely mechanical treatment option that liberates thrombus from the vessel walls and captures in a collection bag to be extracted via the clottriever® sheath. The clottriever thrombectomy system is indicated for: Fda 510(k) clearance received for peripheral thrombectomy by inari | apr 10, 2017 | news The clottriever® system • designed to core, capture and. The clottriever® is a mechanical thrombectomy system designed to remove large clots from large vessels to treat deep vein thrombosis (dvt) in a single session, without the need for. The clottriever® system • designed to core, capture and remove large clots from large venous vessels • in february of 2017, the clottriever system received 510(k). The inari clottriever is a. The inari clottriever is a percutaneous mechanical thrombectomy system consisting of an expandable nitinol collection bag that is dragged along the vein wall,. The clottreiver® system by inari medical® is available to treat the full range of acute and chronic deep vein thrombosis. The clottriever® system is a medical device fda cleared for the treatment of deep vein thrombosis. See instructions for use for complete indications for use,. The clottriever thrombectomy system is indicated for: Fda 510(k) clearance received for peripheral thrombectomy by inari | apr 10, 2017 | news The clottriever® is a mechanical thrombectomy system designed to remove large clots from large vessels to treat deep vein thrombosis (dvt) in a single session, without the need for. In addition, it is designed to give physicians advanced control in chronic. • mechanically core clot from vein wall • capture and remove large clot burden from big vessels • treat in a single session • eliminate. The clottriever® system • designed to core, capture and remove large clots from large venous vessels • in february of 2017, the clottriever system received 510(k). The clottriever® catheter is a purely mechanical treatment option that liberates thrombus from the vessel walls and captures in a collection bag to be extracted via the clottriever® sheath. 2) 2) injection, infusion, and/or aspiration of.(PDF) Percutaneous Thrombectomy of Upper Extremity and Thoracic Central
Inari Medical ClotTriever® Healthcare Essentials
Inari Medical enrolls first patient in ClotTriever trial
Inari Medical VIR ClotTriever November 18, 2020
(PDF) Percutaneous Thrombectomy of Upper Extremity and Thoracic Central
(PDF) Successful removal of an acute deep vein thrombosis by the INARI
Inari 40102 ClotTriever Catheter 11Fr
Inari Medical ClotTriever® Healthcare Essentials
ClotTriever system for removing large clots from large vessels and
ClotTriever system for removing large clots from large vessels and
As The First Mechanical Thrombectomy System To Receive Fda 510 (K) Clearance For The Treatment Of Pulmonary Embolism, The Flowtriever System Is Designed For Rapid Thrombus Removal And.
Inari Medical Announces First Patient Treated With The Clottriever™ Thrombectomy System;
The Clottriever Thrombectomy System Is Intended For Use In The Peripheral Vasculature Including Deep Vein Thrombosis (Dvt).
Related Post: